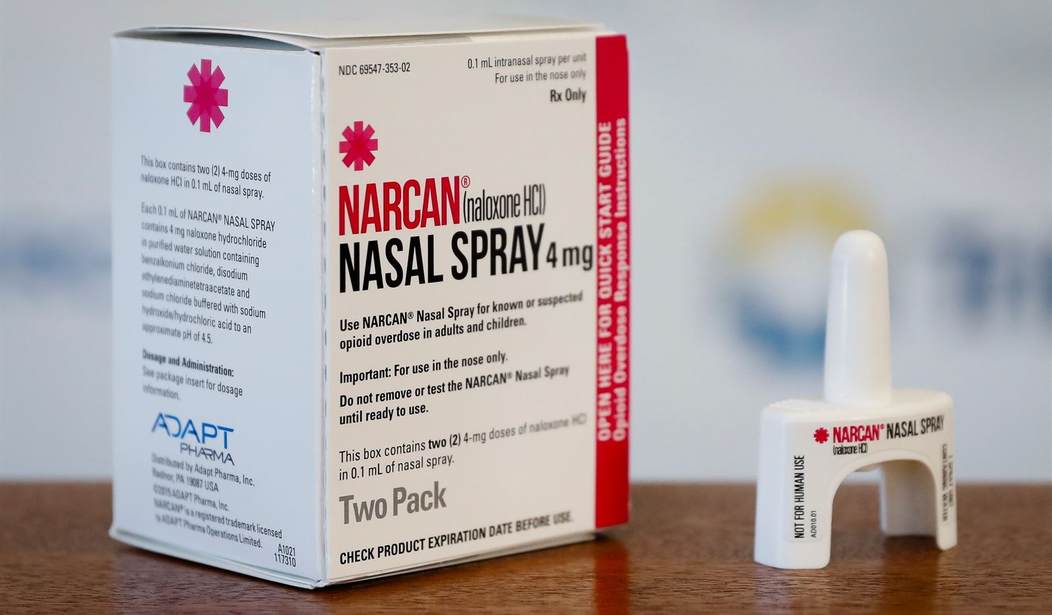
The Wall Street Journal is reporting today that the FDA is fast-tracking approval of an over-the-counter Narcan nasal spray. The FDA has made a push to encourage pharmaceutical companies to apply for approval of over-the-counter opioid-reversal nasal spray because of the fentanyl crisis ravaging communities across America.
In Biden’s America, government actions are reactive, not proactive, so it is refreshing to at least see a push for an over-the-counter version of nasal spray in an effort to move away from prescription-only Narcan. Emergent BioSolutions Inc., the maker of Narcan, said on Tuesday that the FDA has fast-tracked its application. After working on the application for several months, Emergent BioSolutions said the expected approval date is March 29, 2023. This makes the company first in line for approval over competitors.
What is interesting is the pressure being put on pharmaceutical companies to produce an over-the-counter version of nasal spray, as it will eliminate the prescription sprays, unless one has “clinically meaningful difference from nonprescription products.” Otherwise, a product will be “misbranded.”
Last week, FDA Commissioner Robert Califf said naloxone—which binds to opioid receptors to reverse the effects of opioids—should be as ubiquitous as defibrillators.
“Any application we get, this is a high priority. They will get looked at as quickly as we can,” Dr. Califf said.
Pressure from new would-be competitors and the FDA meant Emergent would need to make the switch to over-the-counter. In November, the FDA told prescription drugmakers that once it has sufficient data to support approval of a nonprescription naloxone product, any naloxone products marketed as prescription-only without clinically meaningful difference from nonprescription products will be considered misbranded.
The FDA doesn’t seem to be concerned about Emergent changing its formulation of Narcan – it’s not – but the FDA did ask that people be able to understand the instructions on how to use the product and be able to do so without help from a medical professional. In other words, the FDA wants a consumer to be able to buy Narcan and understand how to use it, as a lay person, at the time of purchase. The question is, will Narcan be any cheaper as an over-the-counter drug? It’s pricey now.
Robert Kramer, president and chief executive of Emergent, said the company hasn’t determined what the price would be for the over-the-counter drug. The average cash price of a box of prescription-only Narcan was $152 in November, according to GoodRx, versus about $137 for generic competitors in the market.
Two other companies seeking over-the-counter versions of naloxone are likely to pressure prices further, as they have said their goal is to slash prices for wider availability. Pocket Naloxone Corp. and Harm Reduction Therapeutics Inc., a nonprofit with funding from now-bankrupt Purdue Pharma LP, both say they aim to make the drug cheap and widely available.
A bipartisan group of lawmakers sent letters to seven major manufacturers of naloxone asking them to make applications for over-the-counter sales.
“As the mental health and addiction crisis continues to plague our nation, this is a monumental step forward for the expansion of more accessible treatments for Americans seeking help,” said Rep. Brian Fitzpatrick (R-Pa.), part of a group that spearheaded the effort and co-chair of the Bipartisan Addiction and Mental Health Task Force.
The opioid crisis, specifically the fentanyl crisis, has become so pronounced that members of Congress are asking drug makers to step up and make their products available without prescriptions. It’s a step in the right direction. Let’s hope that those who need it will be able to afford it. After FDA approval, the next hurdle will be the price.






Join the conversation as a VIP Member